COVID-19 in New Mexico
COVID-19 is a virus that spreads easily. The disease can range from mild to severe. Use all the tools we have available to keep you, your family, and your community safe. Vaccination, testing and treatment can help us all stay safe. And don’t forget to mask up and social distance.
Latest News:
Department of Health to phase out daily COVID-19 Epidemiology Report
SANTA FE – Beginning May 11, in conjunction with the end of the national Public Health Emergency, the New Mexico Department of Health will discontinue its daily reporting of COVID-19 cases, hospitalizations, deaths, and tests. NMDOH will continue to monitor the...
Testing
Knowing if you have COVID-19 can help you seek proper treatment and prevent you from spreading the virus to your family and community.
Vaccine
COVID-19 vaccines are safe and effective. The risk of infection, hospitalization, and death are all much lower for people who are vaccinated, compared to unvaccinated people.
Treatments
Treatment for COVID-19 is recommended for most people who have tested positive for COVID-19.
1-833-796-8773
For COVID-19 Related Questions
1-833-551-0518
For Non-Health Related COVID-19 Questions
Covid.Vaccines@state.nm.us
COVID-19 Related Inquiries
COVID-19 Toolkit
Updated 5-10-2023
We now have many tools to fight COVID: masking, vaccines, treatment, testing, and social distancing. We have reached a new place in New Mexico where we can lift nearly all public health requirements that prevented many COVID cases, hospitalizations, and deaths. It is now possible for us to manage COVID-19 in our homes and in our communities.
This toolkit provides COVID-19 support and guidance for New Mexico communities and individuals.

Table of Contents & Intro

Mask Guidance

Vaccine Guidance

Testing Guidance

Treatment Guidance
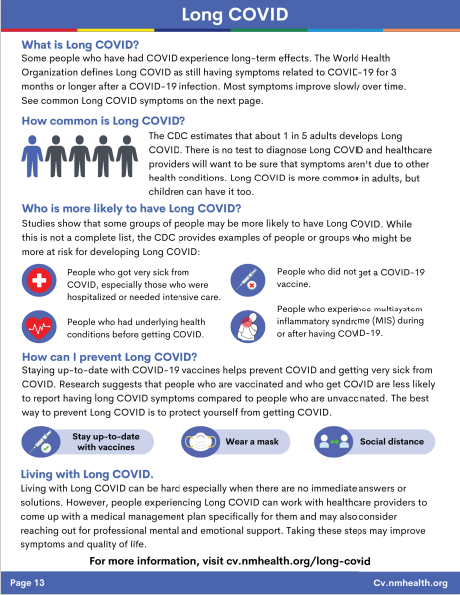
Long COVID Guidance
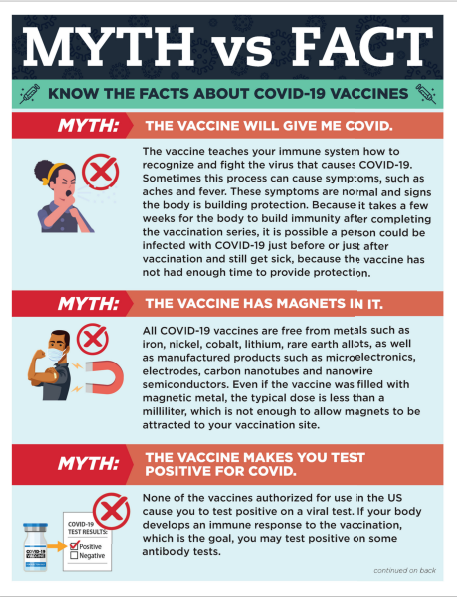
Educational Material

Pregnancy & Breastfeeding Guidance
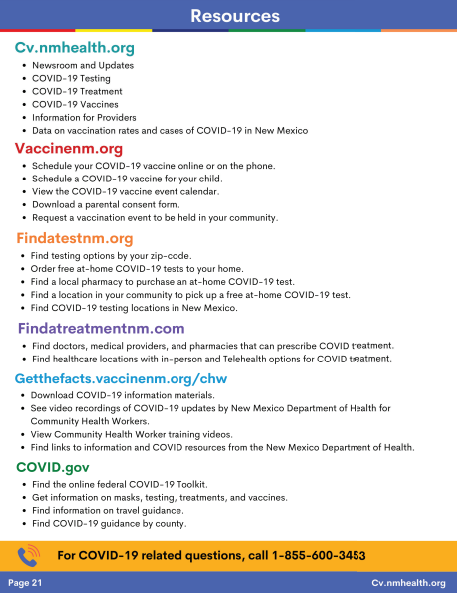
Resources & Links
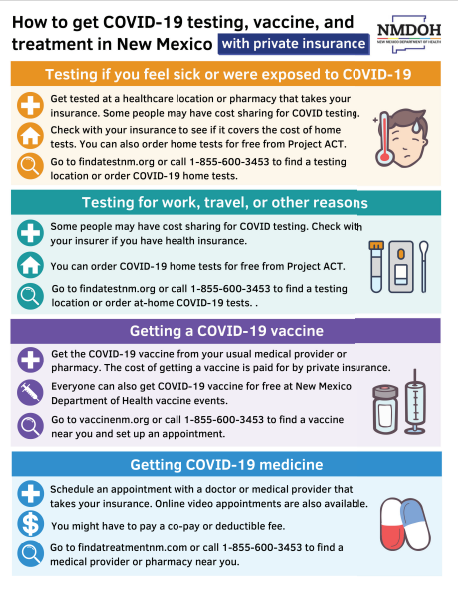
Insurance Info
Latest Medical/Scientific Reports
NMDOH is committed to informing New Mexico residents with the latest data for positive cases, hospitalizations, vaccine rates, and deaths.
Epidemiology Reports
NMDOH collects and analyzes statewide data for COVID-19 positive cases, hospitalizations, and deaths. The reports on this page reflect these critical data and will be updated weekly.
Vaccine Dashboard
The vaccine dashboard currently includes county-by-county breakdowns of vaccine rates throughout the state of New Mexico.
Copyright New Mexico Department of Health


